
Instructions FOr Use
-

Step 1
Apply the enclosed Cavilon Barrier Film wipe to all areas where the tape will be affixed and allow it to dry thoroughly.
Orient the compression pad over the surgical pocket area while centering TOP tape segment 1 between the patient’s neck and shoulder.
Position tape segment 2 beneath the patient’s underarm and tape segment 3 HIGH on the opposite chest wall.
-

Step 2
Holding the compression pad in place, remove liner 1A.
-

Step 3
Holding the compression pad in place, apply the TOP tape segment to the patient. Do NOT overstretch TOP tape segment.
Remove liner segment 1B and apply.
-

Step 4
Holding the compression pad in place, remove liner 2A.
-
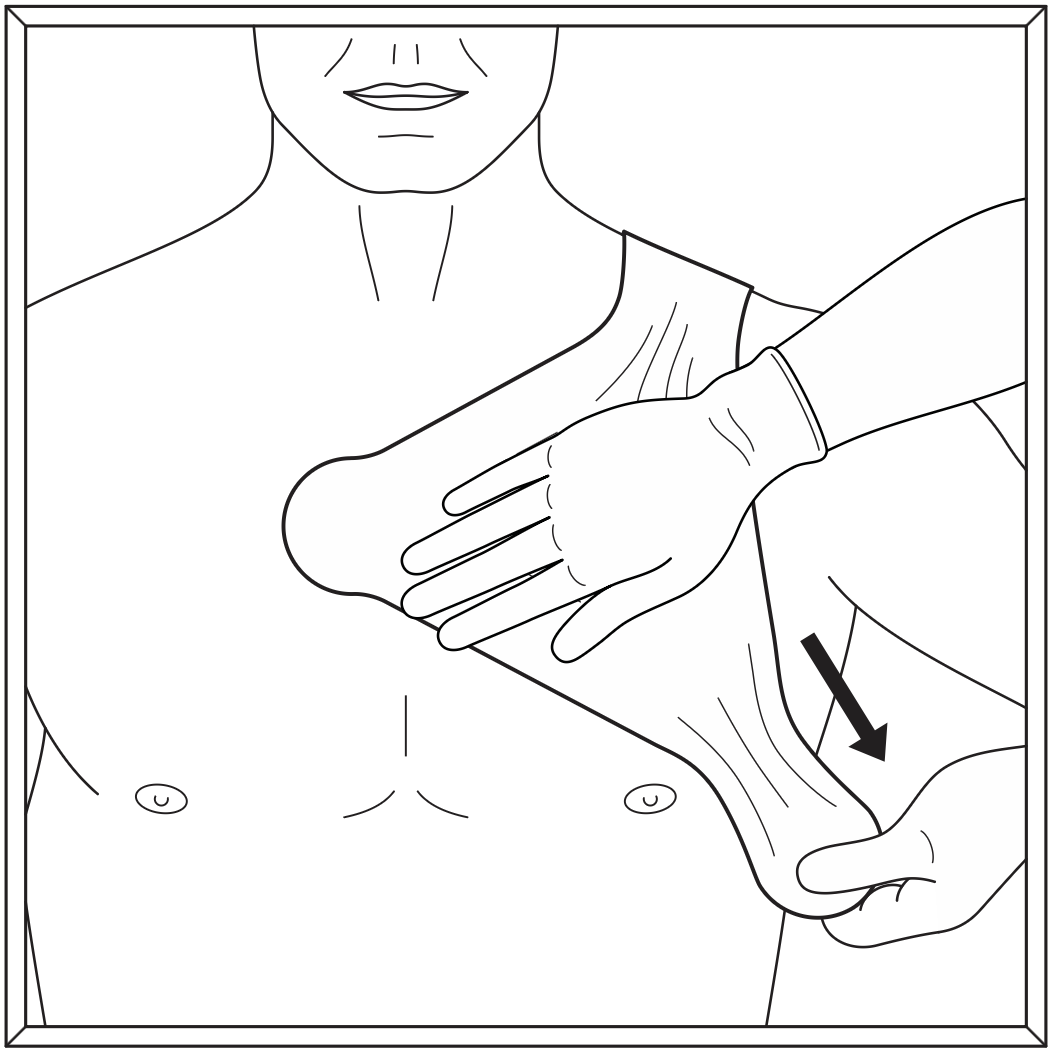
Step 5
Holding the compression pad in place, lightly stretch the axilla tape segment 2 and apply beneath the patient’s underarm.
Remove liner 2B and apply.
-

Step 6
Holding the compression pad in place, remove 3A liner.
Lightly stretch the sternal tape segment 3 and apply high on the opposite chest wall.
Remove liner 3B and apply.
PLACEMENT INSTRUCTIONS
Detailed placement instructions are located on the back of each peel pouch and carton.
Use clean gloves when handling the Pocket Press.
Ensure the surgical pocket has achieved full hemostasis before applying the Pocket Press.
Dress the surgical pocket according to standard practice before applying the Pocket Press Bandage.
Apply Pocket Press only after a sterile primary dressing or skin glue has fully covered the surgical incision and completely dried.
For optimal results, remove as much hair as possible prior to application.
Thoroughly clean and dry application areas.
Use the protective barrier wipe included in pouch and wipe any skin that will be taped prior to use. (The barrier wipe is manufactured by 3M)
Note: Do not allow the Pocket Press to come into direct contact with water.
REMOVAL AND DISPOSAL INSTRUCTIONS
If available, use adhesive remover wipes to assist with removal.
Loosen the edge of the tape,
Stabilize the skin with one finger at the peel line, as the tape is slowly removed, continue to support the skin at the peel line to avoid removal of the primary dressing underneath. If patients will be removing the Pocket Press at home, then provide adhesive remover wipes with their discharge.
Dispose of the Pocket Press in accordance with healthcare facility biohazardous waste protocols.
Federal Law (United States) restricts this device to sale by or on the order of a licensed healthcare provider.
PRODUCT DESCRIPTION
The Pocket Press is a non-sterile pressure bandage intended to be used over a sterile dressing following cardiac implant surgery.
NON-STERILE. Do not open the Pocket Press within a sterile field.
The Pocket Press is an external adhesive bandage made of polyurethane and acrylate adhesive intended to support and compress the surgical pocket following implantation of a pacemaker or defibrillator. It is intended only for left or right-sided cardiac implantable electronic devices (CIED) implants in the pre-pectoral and lateral regions.
The Pocket Press is for use in adults after CIED placement.
The Pocket Press bandage is an elastic polyurethane fabric tape and a polyurethane foam pad.
INTENDED USE
The Pocket Press is intended to be applied over a sterile dressing to apply compression to the surgical pocket, following pacemaker or defibrillator implantation surgery.
INTENDED USER
The Pocket Press is intended to be used by a healthcare professional or by the patient after instruction and under the supervision of a healthcare provider.
INDICATIONS
The Pocket Press is indicated for adult use following pacemaker or defibrillator implantation, which may reduce the incidence of hematomas.
CONTRAINDICATIONS
The Pocket Press is NOT indicated for use in the following situations:
as a sterile pressure bandage.
direct contact with the surgical incision.
ADVERSE REACTIONS
Can cause allergic reactions in persons with skin sensitivity or history of dermatitis. Discontinue use if reaction occurs.
As with any surgical procedure involving the implantation of a pacemaker/defibrillator, there may be complications, including but not limited to, bleeding, hematoma/seroma formation, inflammation, infections, skin tears, extrusion, fistula formation or death. Contact the treating physician immediately should any of these things occur.
WARNINGS / PRECAUTIONS
The Pocket Press is a non-sterile, elastic bandage.
Inspect the packaging to be sure it is intact and undamaged prior to use.
Do not open within a sterile field.
Patients with sensitivity to or history of allergy to polyurethane or acrylate adhesive should not use this tape. Discontinue immediately if sensitivity occurs. Seek treatment per physician guidelines.
The Pocket Press is not indicated for contaminated or infected wounds.
The Pocket Press is not intended to be used as a prophylactic hemostatic agent.
The Pocket Press should only be applied to patients with healthy intact skin.
The Pocket Press should not be allowed to be in direct contact with blood or other bodily fluids.
This device is single-use only and should not be reapplied. The Pocket Press should not be used once the exterior peel pouch has been opened.
Limit wear time of this bandage to 24 hours or less, unless instructed otherwise.
The surgical wound should be inspected per normal guidelines, to assess for any erythema, edema or signs of an infection.
Excessive wear time or not using a skin protective barrier wipe or adhesive remover wipe may make removal more difficult.
Any serious incident that has occurred in relation to the Pocket Press should be reported to the manufacturer and the competent authority of the Member State in which the user and/or patient is established. Incidents may be reported to:
info@pocket-press.com or +1 (929) 444-9115
Cavilon No Sting Barrier Film
WARNINGS
DANGER! HIGHLY FLAMMABLE!
Contains hexamethyldisiloxane which is considered highly flammable.
Cavilon No Sting Barrier Film is highly flammable until it has completely dried on the skin and vapors have dissipated. ALLOW THE PRODUCT TO DRY FOR A MINIMUM OF 90 SECONDS BEFORE USING ANY DEVICE THAT COULD PROVIDE A SOURCE OF HEAT OR IGNITION. THIS ALLOWS FLUID TO DRY AND VAPORS TO DISSIPATE.
Do not use Cavilon No Sting Barrier Film near fire or flame, heat, sparks and sources of static discharge.
Do not use Cavilon No Sting Barrier Film near ignition sources or heat-producing devices. Use in a well-ventilated area.
Wipes are individually packaged for single use only. Reuse could result in increased risk of infection or inadequate product performance.
Keep out of the reach of children.
